Hernia mesh repair devices have been haunting people worldwide for years as their implantation continues to result in severe complications including constant pain, permanent organ problems and sepsis.
More than 1 million Americans undergo hernia repair surgeries each year – one of the most common surgeries performed in the United States, according to the Food and Drug Administration. Among those surgeries, 800,000 are repairs for inguinal hernia – a common type of hernia that protrudes around the groin.
Hernia mesh devices are small tissues made of synthetic materials or animal tissues used for pushing the bulging tissue back into the body and patching it; however, many of the hernia mesh devices on the market are known to shrink or migrate in different parts of the body, causing recurrence and new surgery problems.
Hernia repair is one of the most common surgical procedures. However, the post-surgery complications are usually not considered by the patients. At least 30 percent of patients may suffer from long-lasting chronic pain and restricted movement after the repair surgery.
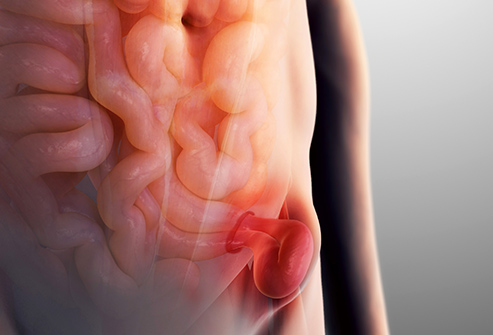
“A hernia occurs when an organ, intestine or fatty tissue squeezes through a hole or a weak spot in the surrounding muscle or connective tissue,” the FDA explains. “Sometimes a hernia can be visible as an external bulge particularly when straining or bearing down.”
Despite the high rate of recurrence in mesh devices, the hernia repair market continues to grow.
Dr. Robert Bendavid, from American Hernia Society, pointed out the issue in an article published in the New Zealand Medical Journal with the question: “If the various mashes were as safe as the industry claims them to be, why are there hundreds of thousands of patients involved in class actions resulting in billions of dollars in fines?”
Mesh device failures are not limited to the U.S. BBC’s Victoria Derbyshire has found in an investigation that up to 170,000 people who have been implanted with hernia mesh tissues in England in the past six years could face complications.
Another horror story recently came from David Ellis, from England, who told iNews that he even has contemplated suicide because of the post-surgery complications. Ellis spent four years in constant pain after the surgery and he is required to take morphine. He cannot sleep more than three hours a night, he said.
It is a paradisematic country, in which roasted parts of sentences fly into your mouth. Even the all-powerful Pointing has no control about the blind texts it is an almost unorthographic life One day however a small line of blind text by the name of Lorem Ipsum decided to leave for the far World of Grammar.
Responsible manufacturers
Among the manufacturers of hernia patch are Ethicon, Versatex, Bard and Atrium. The first recall associated with the hernia mesh device was issued in 2005 for C.R. Bard. In 2013, Atrium’s C-Qur Mesh device was at the center of the issue. The FDA received at least 35 complaints regarding human hair found in the device. In addition, the packaging was defective, and the device would get stuck to the inside lining.
Two years later, Atrium received permanent injunction by the FDA after an investigation indicated that the manufacturer did not address safety violations.
Ethicon’s Physiomesh was designed as an absorbable patch that would be placed in the patient’s abdominal wall. Compared to previous hernia meshes, the new patch would prevent inflammation and adhesions. A Johnson & Johnson subsidiary, Ethicon recalled the Physiomesh patch after receiving a high number of reports regarding the defectiveness of the product. Two studies on Physiomesh revealed that patients had higher incidence of revision surgery or hernia recurrence compared to other products.
Without undergoing clinical tests, Ethicon’s Physiomesh was able to get approval through the 510(k) process – a method that eases the approval of a medical device if it is substantially equivalent to another product already on the market. Since the recall, many Physiomesh patients have taken legal action against Ethicon.
Two years after Ethicon’s recall, the FDA issued another one for Versatex Monofilament Mesh, due to high number of reports on hernia recurrence. Besides the recalls, the agency had previously issued several warnings for Bard, Ethicon and Atrium.
The warning for Bard states the possible danger of breaking ring, which can cause bowel perforation. While Ethicon devices have possible danger of losing the coating of laminate, Atrium’s meshes are packaged improperly.
Legal actions
Christopher Thorpe was awarded $1.3 million by the jury for his injuries and another $200,000 for loss of consortium in 2010. He was implanted with Bard’s Kugel mesh– however, the patch broke inside of Thorpe’s body, causing a serious sepsis infection and internal injuries.
The eyes are on the Physiomesh lawsuit filed by Matthew Huff, who had his surgery in 2013 before experiencing multiple complications associated with the patch. One day Huff went to hospital with fever, nausea, pain and chills. Soon after his doctors discovered the infection, which then resulted in fistula and abscesses in his body. He had to undergo additional surgery.
But Huff took legal action. The 2018 trial was postponed because parties needed additional time.
In August 2018, more than 100 Physiomesh lawsuits were centralized into multicounty litigation (MCL) in New Jersey. Many of the pending lawsuits against mesh producers have been consolidated under multidistrict litigations – one in New Hampshire and the other one in Georgia.

Emre Ertugrul
Emre Ertugrul is a reporter for Safetywatch.org, covering controversial drugs and medical devices, reporting on health policy and the FDA. He studied journalism with concentration in investigative reporting at Boston University. Previous experience with the New England Center for Investigative Reporting include tax issues, racial profiling and criminal justice. He also worked as an international news intern at Milliyet Newspaper and is currently one of the editors for Gazet.com.
Get a Free Hernia Mesh Case Review
If you or a loved one have experienced a severe complication after undergoing hernia mesh implantation surgery, you may be able eligible for compensation.