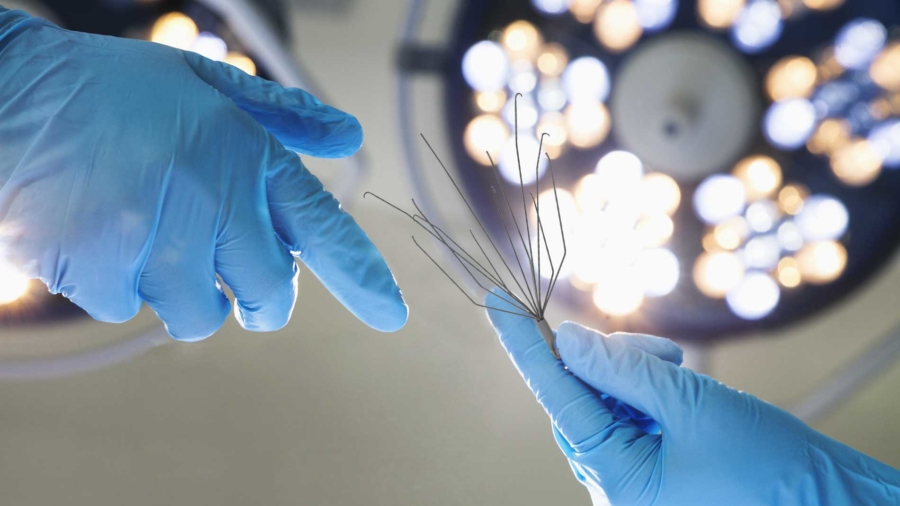
Thousands of patients are routinely endangered by a spider-like medical device implanted in their Inferior Vena Cava, a large vein that is directly connected to the right atrium of the heart.
An estimated 250,000 people in the U.S. go under the knife for IVC filter implantations each year while the liability and dangerous risks of the device have been highly discussed.
Inferior vena cava filters, or simply IVC filters, are manufactured by various companies in different brand names – specially designed for preventing life-threatening blood clots in lung and deep vein thrombosis.
Since the device was approved by the FDA in 1979, several warnings have been released by the agency regarding the risks of injuries and deaths caused by fractures and pieces of the device migrating toward different parts of the system, including the heart.
The filter’s defectiveness can cause clotting and coagulation which then can lead to thrombus and embolism, according to Dr. Ketan Desai, a rheumatologist and published medical author, and the founder of International Medical Consultants.
“The filter itself can embolize, that’s number one,” said Desai. “And number two, if it goes outside the blood vessel, then obviously there is a chance of hemorrhage and infection.”
IVC filters are used when taking blood thinners is not a viable option for a patient. However, the controversy in the manufacture and the FDA reports has raised the doubts on a higher level; in the meantime, some patients died after being implanted with an IVC filter, and hundreds of them were injured.
Among the most reported symptoms of an IVC filter’s adverse effects are nausea, chest pain, heart rhythm anomalies, internal bleeding and pulmonary embolism. In 2007, the FDA has recalled Boston Scientific’s Greenfield IVC filter – which was followed by the death of Cinthia K. Ratliff in 2013, from severe injuries caused by the Greenfield filter. Her case was dismissed after a settlement agreement was reached.
The first IVC filter lawsuit was filed in 2011 against C.R. Bard by Lisa Davis, who had been implanted by a G2 series IVC filter in 2006. The device fractured over time and migrated to her heart in 2008. A settlement was reached between the manufacturer and the plaintiff in 2013.
The majority of the IVC filter lawsuits have been filed against C.R. Bard and Cook Medical on the grounds that manufacturers were aware of the defectiveness of their devices and failed to inform doctors and patients about the risks.
There are three pending class action suits against Bard to be held in California, Florida and Pennsylvania. Plaintiffs claim that the company was negligent and concealing data from intermediaries or patients. One of the significant points of the claims is that even if the IVC filters did not migrate or fracture, they caused serious problems for patients that they have been entitled to routine medical monitoring. As of May 2018, 3,834 class action lawsuits were pending against Bard. The number is 4,189 for Cook Medical.
The first bellwether trial for Bard was held in Arizona in March 2018. The federal jury awarded a verdict of $3.6 million to plaintiff Sherr-Una Booker, who suffered health problems and underwent a heart surgery after a Bard G2 filter had been implanted in 2007.lamcorper mattis, pulvinar dapibus leo.
After the first two IVC filter lawsuits against Cook resulted in a defendant’s verdict, the third verdict was announced on May 29, 2018 in Texas – awarding $1.2 million to plaintiff Jeff Pavlock, who claimed in his case that he suffered vessel and organ perforations after a Cook Celect filter was implanted in 2015. According to doctors, Pavlock requires lifetime health monitoring due to the complications he had suffered.
According to a 2012 research published on JAMA Network, another controversial inferior vena cava filter, Cordis’ TrapEase, showed a 50 percent rate of fracture in patients 50 months after the implantation. After four years, the rate went up to 64 percent.
In 2013, Cordis’ OptEase IVC filter met a Class I FDA medical device recall after the company provided medical professionals with information regarding the serious risks in implanting the filter.
According to an NBC investigation of 2015, medical device giant C.R. Bard held a huge revenue from its sales of 34,000 Recovery filters even after the serious risks associated with the filter were revealed. In 2005, a new model called “the G2” replaced Recovery.
NBC’s report stated that Bard’s filters were associated with 27 deaths and hundreds of adverse events. The report also revealed a controversial paperwork process in attempts to gain FDA clearance to sell the Recovery filter. At the end, NBC raised the question “Did Forged Signature Clear Way for Dangerous Blood-Clot Filter?” in a September 2015 report.
Under the 510(k) process, when a medical device has been approved based on another “substantially equivalent” device, the FDA does not have to look at whether or not the formerly-approved predicate device was unsafe. The only point the agency follows is to see if the device is being requested for clearance.
According to Madris Tomes, founder and CEO of Device Events, there can be devices that got recalled and are no longer on the market, but something else can be approved based on that device.
Bard’s G2 filter, meaning “generation two,” replaced the original Recovery filter in 2005. In 2017, medical technology company Becton Dickinson announced its acquisition of Bard.
“There were physicians who submitted the reports about the Recovery device and the issues that they had,” said Tomes, who is also a former FDA official. “And when the newer device G2 came out, there was an assumption on the physicians’ part that the problems they had been reporting about the Recovery device have been fixed.”
She said there was no way for those physicians to know if the representatives were not telling them that very little change between Recovery and the G2.
“It was a very tricky way to name the new device,” Tomes said. “And you’re seeing just as many, if not, more reports on the G2 than on the Recovery filter.”
Among the adverse event reports that Device Events has provided, there are 695 death files associated with an IVC filter treatment reported by manufacturers, attorneys, users and physicians.
One of the reports, which was filed on Sept. 6, 2018, states that approximately two years after the implant, a patient went under the knife because of the migrated portions of TrapEase. The portions were surgically removed from the heart. Findings included “three pieces of metal wires, the largest of which has an incomplete umbrella-like configuration with two pointed ends.”
One month after the surgery, the patient died.
Another patient died in the hospital while taken in comfort care after a second IVC filter was placed above the thrombus that was discovered free floating in the pre-existing filter. The May 2017 report states that the death certificate documented the cause of death as “upper gastrointestinal bleed with pulmonary embolus and thrombus from the inferior vena cava filter.”
Although Bard, the manufacturer, conducted investigation on its Denali Jugular System’s device history records, nothing was found to indicate that the death was related to manufacturing.
However, as the device was not returned to the manufacturer, a visual inspection could not be performed. In most of the death cases, products have not been returned for analysis – meaning that the manufacturer could not confirm the filter complications without procedural films.
An OptEase filter was found in a patient’s heart after the patient had a cardiac incident and died ten days after the filter deployment. In the November 2010 report, Cordis, the manufacturer, states that there was not enough information to draw “a definitive clinical conclusion” linked with the device.
In some reports, because lot numbers and catalog numbers are not provided, the brand name is listed as “unknown.” However, in a March 2018 injury report, manufacturer William Cook Europe states that the device was referred to as a Cook Celect.
In some cases, if physicians used the FDA MAUDE adverse event system in order to review patients with a certain problem associated with a particular device, they would have had to type “unknown” in order to look up the device, according to Madris Tomes. Otherwise, they would not have found the problem.
“There is a lack of transparency even when the device report is made public,” she said. “If you don’t know how to find it or how to look for the report, you’re going to have a lot of difficulty.”
*The FDA adverse event reports mentioned in this article were provided by Device Events for analysis and identification.
Written by:

Emre Ertugrul
Emre Ertugrul is a reporter for Safetywatch.org, covering controversial drugs and medical devices, reporting on health policy and the FDA. He studied journalism with concentration in investigative reporting at Boston University. Previous experience with the New England Center for Investigative Reporting include tax issues, racial profiling and criminal justice. He also worked as an international news intern at Milliyet Newspaper and is currently one of the editors for Gazet.com.
Get a Free IVC Filter Case Review
If you or a loved one experienced a complication after getting an IVC filter, you may be eligible for money to pay for health care costs.